Question 1.
Name the simplest hydrocarbon. (AS1)
Answer:
The simplest hydrocarbon is alkane called Methane (CH4). It’s an aliphatic, saturated compound of Hydrogen and Carbon.
Question 2.
What are the general molecular formulae of alkanes, alkenes and alkynes? (AS1)
Answer:
General molecular formula of alkane is CnH2n+2.
General molecular formula of alkene is CnH2n.
General molecular formula of alkyne is CnH2n-2.
Question 3.
Name the carboxylic acid used as a preservative. (AS1)
Answer:
Vinegar with chemical formula CH3COOH is used as preservative. 5 – 8% of solution of acetic acid or ethanoic acid in water is called vinegar and it is used widely as preservative in pickles.
Question 4.
Name the product other than water formed on burning of ethanol in air. (AS1)
Answer:
C2H3OH + 3O2→ 2CO2+ 3H2O + Energy
So, the product other than water formed on burning of ethanol in air is carbon dioxide (CO2).
Question 5.
Give the IUPAC name of the following compounds. If more than one compound is possible, name all of them. (AS1)
i) An aldehyde derived from ethane.
ii) A ketone derived from butane.
iii) A chloride derived from propane.
iv) An alcohol derived from pentane.
Answer:
i) An aldehyde derived from ethane is ethanal. Its formula is CH3CHO.
ii) A ketone derived from butane. Its IUPAC name is Butanone.
Its chemical formula is CH3COCH2CH3
It is also known as methyl ethyl ketone. (Its general name)
iii) A chloride derived from propane.
A) 1-Chloro propane. Its formula is CH3CH2CH2Cl.

iv) An alcohol derived from pentane :
A) 1-Pentanol. Its formula is CH3CH2CH2CH2CH2OH.
B) 2-Pentanol. Its formula is CH3CHOH CH2CH2CH3
C) 3-Pentanol. Its formula is CH3CH2CHOH CH2CH3
Question 6.
A mixture of oxygen and ethyne is burnt for welding ; can you tell why a mixture of ethyne and air is not used? (AS1)
Answer:
- Ethyne when burnt in the presence of oxygen gives enough heat that can be used for welding.
- Whereas if it is burnt in air which contains nitrogen, CO2and other inactive gaseous contents, sufficient oxygen is not available for burning ethyne to give the required heat.
Question 7.
Explain with the help of a chemical equation, how an addition reaction is used in vegetable ghee industry. (AS1)
Answer:
- The addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated hydrocarbon is called hydrogenation. The process of hydrogenation takes place in the presence of nickel or palladium metals as catalyst.
- The process of hydrogenation has an important industrial application. It is used to prepare vegetable ghee (or vanaspati ghee) from vegetable oils.
- Vegetable oils are unsaturated fats having double bonds between some of their carbon atoms.
- When a vegetable oil (like groundnut oil) is heated with hydrogen in the presence of finely divided nickel as catalyst, a saturated oil called vegetable ghee (or vanaspati ghee) is formed. This a reaction is called hydrogenation of oils and it can be represented as follows.
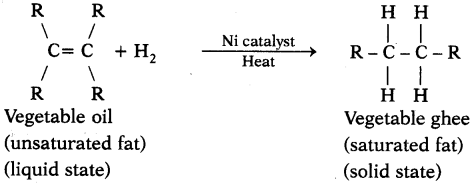
Here vegetable oil is a liquid whereas vegetable ghee is a solid (or a semi solid).
Question 8.
a) What are the various possible structural formulae of a compound having molecular formula C3H6O? (AS1)
b) Give the IUPAC names of the above possible compounds and represent them in structures. (AS1)
c) What is the similarity in these compounds? (AS1)
Answer:
a) They are CH3COCH3and CH3CH2 CHO
b) i) The IUPAC name of CH3COCH3is propanone.
ii) The IUPAC name of CH3CH2CHO is propanal.
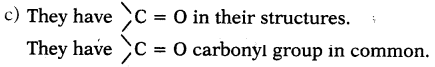
Question 9.
Name the simplest ketone apfl write its molecular formula. (AS1)
Answer:
Acetone is the simplest ketone. Its molecular formula is CH3COCH3Its IUPAC name is propanone.
Question 10.
What do we call the Self linking property of carbon? (AS1)
Answer:
The property of self combination (or linking) of carbon atoms to form long chains is useful to us because it gives rise to an extremely large number of carbon compounds (or organic compounds). This is known as catenation.
Question 11.
Name the compound formed by heating ethanol at 443 K with excess of cone. H2SO4. (AS1)
(OR)
What is the compound formed when ethyhalcohol (Ethanol) is dehydrated ? Write the chemical equation of the reaction.
Answer:
1. When ethanol is heated with excess of cone. H2SO4at 443 K (170° C), it gets dehydrated to form ethene (which is an unsaturated hydrocarbon).
2. During dehydration of ethanol molecules (CH3- CH2OH), H from the CH3group and OH from CH2OH group are removed in the form of a water molecule (H2O) regulating in the formation of this molecule (CH2= CH2).
3. In this reaction concentrated sulphuric acid acts as a dehydrating agent.
Question 12.
Give an example for esterification reaction. (AS1)
Answer:
The reaction between carboxylic acid and an alcohol in the presence of cone. H2SO4to form a sweet odoured substance, ester with the functional group
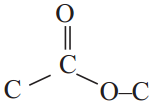
is called esterification.
Ex: Ethanoic acid (carboxylic acid) reacts with Ethanol (alcohol) and forms ethyl acetate.
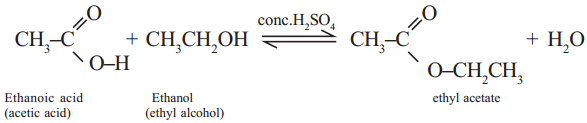
Question 13.
Name the product obtained when ethanol is oxidized by either chromic anhydride or alkaline potassium permanganate. (AS1)
(OR)
If the ethanol is oxidized by either chromic anhydride or alkaline potassium permanganate, what is the product obtained from them?
Answer:
Ethanol (Ethyl alcohol) undergoes oxidation to form the product of Acetaldehyde and finally Acetic acid.
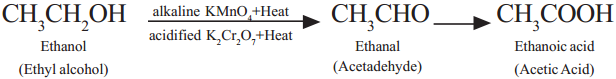
Question 14.
Write the chemical equation representing the reaction of preparation of ethanol from ethane. (AS1)
Answer:
1. Ethane in the absence of air on heating forms ethene
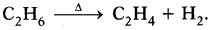
2. Then Ethanol is prepared on large scale from ethene by the addition of water vapour to it in the presence of catalyst like P2O5, Tungsten oxide at high pressure and temperature.

Question 15.
Write the IUPAC name of the next homologous of CH3OHCH2CH3. (AS1)
Answer:
The IUPAC name of the next homologous of CH3OHCH2CH3is HO-CH3CH2CH2CH31 - butanol.
Question 16.
Define homologous series of carbon compounds. Mention any two characteristics of homologous series. (AS1)
Answer:
1. The series of carbon compounds in which two successive compounds differ by – CH2unit is called homologous series.
Ex : 1) CH4, C2H6, C3H8, ………………..
2) CH3OH, C2H5OH, C3H7OH, ………………..
2. If we observe above series of compounds, we will notice that each compound in the series differs by – CH2unit by its successive compound.
3. Characteristics of homologous series :
i) They have one general formula.
Ex : alkanes (CnH2n+2), alkynes (CnH2n-2), alcohols (CnH2n+1) OH, etc.
ii) Successive compounds in the series possess a difference of (-CH2) unit.
iii) They have similar chemical properties.
Question 17.
Give the names of functional groups
(i) - CHO
(ii) - C = O. (AS1)
(OR)
Write the names of the given functional groups
(i) - CHO
(ii) - C = O
Answer:
i) - CHO → aldehyde
ii) - C = O → ketone
Question 18.
Why does carbon form compounds mainly by covalent bonding? (AS1)
Answer:
Since carbon atoms can achieve the inert gas electron arrangements only by the sharings of electrons, therefore, carbon always forms covalent bonds.
Question 19.
Allotropy is a property shown by which class substance: elements, compounds or mixtures? Explain allotropy with suitable examples. (AS1)
Answer:
1. Allotropy is a property shown by the elements.
2. The property of an element to exist in two or more physical forms having more or less similar chemical properties but different physical properties is called allotropy.
3. The different forms of the element are called allotropes and are formed due to the difference in the arrangement of atoms.
4. Example for allotropes : Allotropes of carbon.
Allotropes of carbon are classified into two types. They are
1) Amorphous forms,
2) Crystalline forms.
5) Amorphous forms of carbon:
Coal, coke, wood, charcoal, animal charcoal, lampblack, gas carbon, petroleum coke, sugar charcoal.
6) Crystalline forms of carbon :
Diamond, graphite and buckminsterfullerene.
Question 20.
Explain how sodium ethoxide is obtained from ethanol. Give chemical equations. (AS1)
Answer:
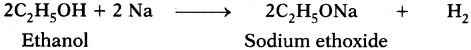
As ethanol is similar to water molecule (H2O) with C2H5group in place of hydrogen, it reacts with metallic sodium to liberate hydrogen and form sodium ethoxide.
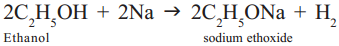
Question 21.
Describe with chemical equation how ethanoic acid may be obtained from ethanol. (AS1)
Answer:
Ethyl alcohol (Ethanol) undergoes oxidation to form the product Acetaldehyde and finally acetic acid (Ethanoic acid).
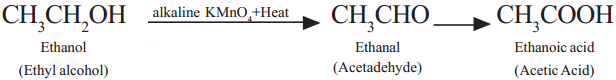
Question 22.
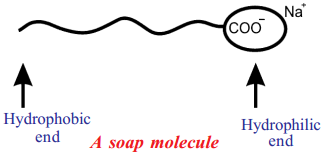
Explain the cleansing action of soap. (AS1)
Answer:
When a dirty cloth is put in water containing dissolved soap, the hydrocarbon ends of the soap molecules in the micelle attach to the oil or grease particles present on the surface of dirty clothes.
Question 24.
Explain the structure of graphite in terms of bonding and give one property based on this structure. (AS1)
(OR)
Why does graphite act as lubricant?
Answer:
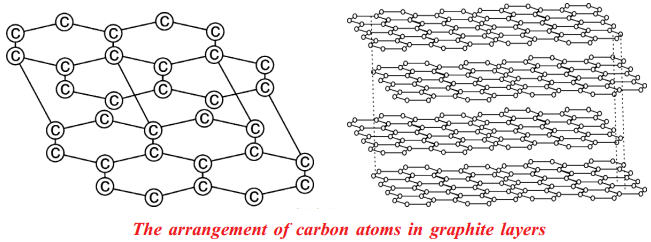
- Graphite forms a two dimensional layer structure with C – C bonds within the layers.
- There are relatively weak interactions between the layers.
- In the layer structure, the carbon atoms are in a trigonal planar environment.
- This is consistent with each carbon atom in sp2 hybridisation.
- Interactions between the sp2 orbitals (overlaps) lead to the formation of C - C bonds.
- Each carbon atom is with one unhybridised ‘p’ orbital.
- The unhybridised ‘p’ orbitals interact to form a π system that is delocalised over the whole layer.
- The interactions known as London dispersion forces between the layers which are separated by a distance of 3.35 A° are weakened by the presence of water molecules so that it is easy to cleave graphite.
- For this reason graphite is used as lubricant and as the lead in pencils.
Question 25.
Name the acid present in vinegar. (AS1)
Answer:
1) The acid present in vinegar is Ethenoic acid or acetic acid (CH3COOH).
2) 5 - 8% solution of acetic acid in water is called vinegar.
Question 26.
What happens when a small piece of sodium is dropped into ethanol? (AS2)
Answer:
Ethanol reacts with sodium to liberate hydrogen and form sodium ethoxide.
Question 27.
Two carbon compounds A and B have molecular formula C3H8and C3H6respectively. Which one of the two is most likely to show addition? Justify your answer. (AS2)
Answer:
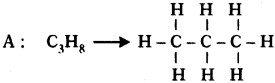
. It is a saturated hydrocarbon. It shows substitution reaction.
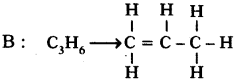
This is an unsaturated hydrocarbon. Hence it shows addition to become saturated. During the reactions, addition of reagent takes place at the double bonded carbon atoms.
Justification :
In the following, C3H6 undergoes addition reaction.
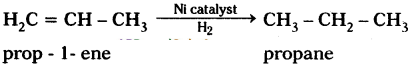
Question 28.
Suggest a test to find the hardness of water and explain the procedure. (AS3)
(OR)
How do you test whether a given water sample is soft or hard?
Answer:
- Take about 10 ml hard water (well water or hand pump water) in a test tube.
- Add five drops of soap solution to it.
- Shake the test tube vigorously.
- We see that no lather is formed at first.
- Only a dirty white curd like scum is formed on the surface of water.
- From this, we conclude that soap does not form lather easily with hard water.
- We have to add much more soap to obtain lather with hard water.
Question 29.
Suggest a chemical test to distinguish between ethanol and ethanoic acid and explain the procedure. (AS3)
Answer:
- Take ethanol and ethanoic acid in two different test tubes.
- Add nearly 18 g of sodium bicarbonate (NaHCO3) to each test tube.
- Lots and lots of bubbles and foam will be observed from the test tube containing ethanoic acid. This is due to release of CO2.
NaHCO3+ CH3COOH → CH3COONa + H2O + CO2
- Ethanol will not react with sodium bicarbonate and thus we won’t observe any change in the test tube containing ethanol.
Thus we can separate ethanol from ethanoic acid.

Question 30.
An organic compound ‘X’ with a molecular formula C2H6O undergoes oxidation with alkaline KMnO4 and forms the compound ‘Y’, that has molecular formula C2H4O2. (AS3)
i) Identify ‘X’ and ‘Y’.
Answer:
X is Ethanol is CH3CH2OH and T is Ethanoic acid, i.e., CH3COOH.
ii) Write your observation regarding the product when the compound X is made to react with compound IT which is used as a preservative for pickles.
Answer:
Ethyl alcohol undergoes oxidation to form the product Acetaldehyde and finally Acetic acid.

Here CH3COOH is used as preservative for pickles.
When X reacts with Y it forms ethyl acetate and water which is called esterification reaction.
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
Question 31.
Prepare models of methane, ethane, ethene and ethyne molecules using clay balls and matchsticks. (AS4)
Answer:
Stick and ball model :
1) Methane (CH4) :
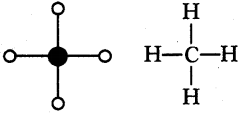
2) Ethane (C2H6):
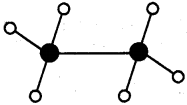
3) Ethene (C2H4):
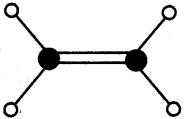
4) Ethyne (C2H2)
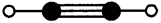
Question 32.
Collect information about artificial ripening of fruits by ethylene. (AS4)
Answer:
- Seasonal fruits like mango, banana, papaya, sapota and custard apple are often harvested in nature. But due to unripe condition they are subsequently allowed to ripen by natural release of ripening harmone (ethylene) from the fruit.
- However, natural ripening in some fruits is a slow process, which leads to high weight loss, desiccation of fruits and under ripening. With the rapid development of fruit trade, artificial ripening has become essential and the methods practised earlier by small traders are smoking and calcium carbide treatment.
- Fruits ripened with calcium carbide though seem attractive and colourful are inferior in taste, flavour and spoil faster.
- Government of India has banned the use of calcium carbide for artificial ripening of fruits under PFA Act 8-44AA, 1954.
- Artificial ripening of fruits by using the above steps spoils the health of consumers, so we should not use such type of fruits.
- Government has to take serious action on the fruit sellers who are practising the above said methods.
Question 33.
Draw the electronic dot structure of ethane molecule (C2H6). (AS6)
Answer:
C2H6:
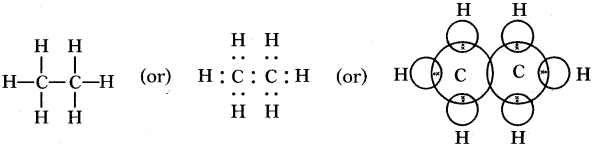
Question 34.
How do you appreciate the role of esters in everyday life? (AS6)
Answer:
- Esters are usually volatile liquids having sweet or pleasant smell.
- They are also said to have fruity smell.
- Esters are used in making artificial perfumes.
- This is because of the fact that most of the esters have a pleasant smell.
- Esters are also used as flavouring agents.
- This means that esters are used in making artificial flavours and essences used in ice-cream, sweets and cool drinks.
- The alkaline hydrolysis of esters is known as saponification (Soap making).
- That’s why we can appreciate the role of esters in everyday life.
Question 35.
How do you condemn the use of alcohol as a social practice? (AS7)
Answer:
- Consumption of alcohol in the form of beverages is harmful to health.
- It causes severe damage to blood circulation system.
- Addiction to alcohol drinking leads to heart diseases and damages the liver.
- It also causes ulcers in small intestines due to increased acidity and damages the digestive system.
- Alcohol which is consumed in raw form under the names liquor, gudumba which is more harmful to health due to adulteration.
- Alcohol mixed with pyridine is called denatured spirit. Consumption of denatured spirit causes blindness and death.
- Hence use of alcohol is a social evil which harms the society.
Question 36.
An organic compound with molecular formula C2H4O2produces brisk effervescence on addition of sodium carbonate/bicarbonate.
Answer the following :
a) Identify the organic compound. (AS1)
Answer:
The organic compound is Ethanoic acid (CH3COOH).
b) Write the chemical equation for the above reaction. (AS1)
Answer:
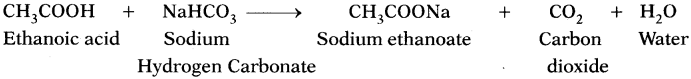
c) Name the gas evolved. (AS2)
Answer:
CO2
d) How will you test the gas evolved? (AS3)
Answer:
1) Pass the evolved gas through lime water in a test tube.
2) We will find that lime water turns milky.
3) Only CO2 gas can turn lime water milky.
e) List two important uses of the above compound. (AS1)
Answer:
1) Dilute ethanoic acid (CH3COOH) is used as a food preservative in the preparation of pickles and sauces.
2) Ethanoic acid is used for making cellulose acetate which is an important artificial fibre.
Question 37.
1 ml glacial acetic acid and 1 m/of ethanol are mixed together in, a test tube. Few drops of concentrate sulphuric acid is added in the mixture are warmed in a water bath for 5 min.
Answer the following:
a) Name the resultant compound formed.
b) Represent the above change by a chemical equation.
c) What term is given to such a reaction?
d) What are the special characteristics of the compound formed?
Answer:
a) Ethyl acetate.
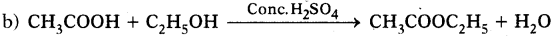
c) Esterification
d) It has fruity smell or pleasant smell.